Achieves world’s highest power output for passive-type bio batteries
Sony today announced the development of a bio battery1 that generates electricity from carbohydrates (sugar) utilizing enzymes as its catalyst, through the application of power generation principles found in living organisms.
Test cells of this bio battery have achieved power output of 50 mW, currently the world’s highest level2 for passive-type3 bio batteries. The output of these test cells is sufficient to power music play back on a memory-type Walkman.
4 prototype bio battery units (left) connected to Walkman for playback
In order to realize the world’s highest power output, Sony developed a system of breaking down sugar to generate electricity that involves efficiently immobilizing enzymes and the mediator (electronic conduction materials) while retaining the activity of the enzymes at the anode. Sony also developed a new cathode structure which efficiently supplies oxygen to the electrode while ensuring that the appropriate water content is maintained. Optimizing the electrolyte for these two technologies has enabled these power output levels to be reached.
Sugar is a naturally occurring energy source produced by plants through photosynthesis. It is therefore regenerative, and can be found in most areas of the earth, underlining the potential for sugar-based bio batteries as an ecologically-friendly energy device of the future.
Sony will continue its development of immobilization systems, electrode composition and other technologies in order to further enhance power output and durability, with the aim of realizing practical applications for these bio batteries in the future.
The research results presented here have been accepted as an academic paper at the 234th American Chemical Society National Meeting & Exposition in Boston, MA USA, and were announced at 11 am local time on August 22, 2007.
The bio battery mechanism
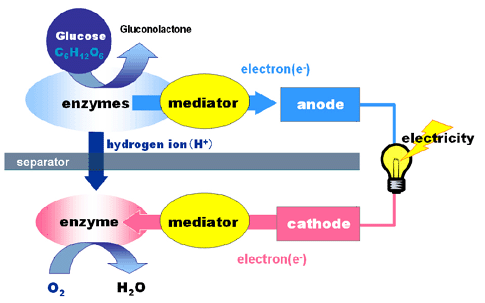
The newly developed bio battery incorporates an anode consisting of sugar-digesting enzymes and mediator, and a cathode comprising oxygen-reducing enzymes and mediator, either side of a cellophane separator. The anode extracts electrons and hydrogen ions from the sugar(glucose) through enzymatic oxidation as follows:
Glucose -> Gluconolactone + 2 H+ + 2 e–
The hydrogen ion migrates to the cathode through the separator. Once at the cathode, the hydrogen ions and electrons absorb oxygen from the air to produce water:
(1/2) O2 + 2 H+ + 2 e– -> H2O
Through this process of electrochemical reaction, the electrons pass through the outer circuit to generate electricity.
Key achievements of this bio battery research and development
1) Technology to enhance immobilization of enzymes and mediator on the electrode
For effective glucose digestion to occur, the anode must contain a high concentration of enzymes and mediator, with their activity retained. This technology uses two polymers to attach these components to the anode. Each polymer has opposite charge so the electrostatic interaction between the two polymers effectively secures the enzymes and mediator. The ionic balance and immobilization process have been optimized for efficient electron extraction from the glucose.
2) Cathode structure for efficient oxygen absorption
Water content within the cathode is vital to ensuring optimum conditions for the efficient enzymatic reduction of oxygen. The bio battery employs porous carbon electrodes bearing the immobilized enzyme and mediator, which are partitioned using a cellophane separator. The optimization of this electrode structure and process ensures the appropriate water levels are maintained, enhancing the reactivity of the cathode.
3) Optimization of electrolytes to meet the bio battery cell structure
A phosphate buffer of approximately 0.1 M is generally used within enzymology research, however an unusually high 1.0 M concentration buffer is used in this bio battery. This is based on the discovery that such high concentration levels are effective for maintaining the activity of enzymes immobilized on the electrodes.
4) Test cell combining high-power output and compact size
The test cells of these high-power, compact bio batteries have been fabricated using these three technologies. The bio battery does not require mixing, or the convection of glucose solution or air; as it is a passive-type battery, it works simply by supplying sugar solution into the battery unit. The cubic (39 mm along each edge) cell produces 50 mW, representing the world’s highest power output among passive-type bio batteries of comparable volume. By connecting four cubic cells, it is possible to power a memory-type Walkman (NW-E407) together with a pair of passive-type speakers (no external power source). The bio battery casing is made of vegetable-based plastic (polylactate), and designed in the image of a biological cell.
Bio battery technical specifications
| Enzymes | Glucose dehydrogenase and diaphorase (anode) Bilirubin oxidase (cathode) |
| Mediators | Vitamin K3 and cofactor NADH (anode) Potassium ferricyanide (cathode) |
| Electrode | Porous carbon |
| Current collector | Titanium mesh |
| Separator | Cellophane |
| Glucose solution | 0.4 M glucose in 1.0 M sodium phosphate buffer, pH 7.0 |
| Maximum output | 1.5 mW/cm2 (0.3V, 5 mA/cm2) at 1 min after connection |
| OCV | 0.8V |
Bio battery test cell specifications
| Dimensions | 39 (width) x 39 (height) x 39 (depth) mm |
| Volume | 40 cc (without casing) |
| Maximum output | 50 mW |
Reference
- Bio battery: An electricity generation device that utilizes energy sources such as carbohydrates, protein, amino acids, fat by digesting enzymes. Since 2001, Sony’s research has been supported by Professor Kenji Kano’s laboratory (formerly professor Tokuji Ikeda’s laboratory) at the Division of Applied Life Sciences, Graduated School of Agriculture, Kyoto University, Kyoto, Japan, which specializes in bioelectrochemistry. The results presented here are based on Sony’s original technological developments, inspired by the lab’s advanced research activities.
- 50 mW: World’s highest power output as of August 23, 2007, based on Sony research.
- Passive-type battery: A system in which reactive substances such as glucose and oxygen are absorbed into electrodes through a process of natural diffusion. In contrast, systems in which reactive substances are supplied by force (stirring, convection) are referred to as “active-type”. In general, passive-type systems have a more simple structure suitable for miniaturization, whereas active type systems have a more complicated structure and are suited to higher power devices.




















